Developing five-membered heterocycle substituted phosphinous acids as ligands for palladium-catalyzed Suzuki–Miyaura and Catellani reactions†
Abstract
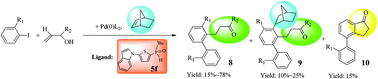
A new category of secondary phosphine oxides (SPOs) (5a–5j) with/without benzo-fused five-membered heterocyclic substituents were prepared. These new compounds are air- and moisture-stable ligands and have the advantage of long-term storage. Some of the ligands as well as ligand coordinated palladium complexes (6f′ and 6f′′) and platinum complexes (7b_trans & 7i_trans) were prepared and their structures were determined using single crystal X-ray diffraction methods. The crystal structure of 6f′ revealed the formation of diamond shape di-palladium complexes with a Pd2Cl2 core. As for the structures of 7b_trans & 7i_trans, the processes for the generation of the trans-form of the bis-phosphine ligand coordinated platinum complexes are shown. These SPOs exhibit notable efficiencies in palladium-catalyzed Suzuki–Miyaura reactions. Moreover, organic compounds (9k and 10c) with unexpected conformations were obtained from Heck-type Catellani reactions. Reaction pathways are proposed to accommodate the probable routes for the formation of all organic products.

 Please wait while we load your content...
Please wait while we load your content...