Carboxylic acid derivatives via catalytic carboxylation of unsaturated hydrocarbons: whether the nature of a reductant may determine the mechanism of CO2 incorporation?†
Abstract
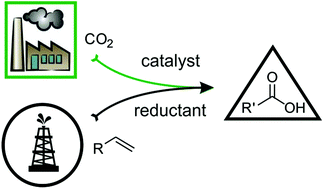
Application of CO2 as a renewable feedstock and C1 building block for production of commodity and fine chemicals is a highly challenging but obvious industry-relevant task. Of particular interest is the catalytic coupling of CO2 with inexpensive unsaturated hydrocarbons (olefins, dienes, styrenes, alkynes), providing direct access to carboxylic acids and their derivatives. Although not brand new for the scientific community, it is still a complete challenge, as no truly effective catalytic system has been reported to date. In this Perspective, we discuss the available experimental, theoretical and mechanistic data for such homogeneously catalyzed carboxylation processes. A special focus is placed on the understanding of the key elementary steps and of some thermodynamic and kinetic constraints.

 Please wait while we load your content...
Please wait while we load your content...