Fluoride-free Hiyama coupling by palladium abnormal N-heterocyclic carbene complexes†
Abstract
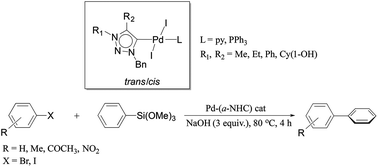
A series of palladium complexes of the abnormal N-heterocyclic carbene ligands of the type (a-NHC)PdI2(L) [L = NC5H5(1–3)b and PPh3(1–3)c] effectively catalyzed the Hiyama coupling of aryl bromides and iodides with PhSi(OMe)3 under the highly desired fluoride-free conditions. Interestingly enough, the pyridine based trans-(1–3)b complexes and a PPh3 derived cis-3c complex exhibited higher yields than the related PPh3 derived trans-(1–2)c complexes. The superior performances of the pyridine based trans-(1–3)b complexes and the PPh3 derived cis-3c complex have been correlated to a tighter binding of the a-NHC ligand to the palladium center in these complexes, leading to a greater (a-NHC) ligand influence on the metal center partaking in the catalysis.


 Please wait while we load your content...
Please wait while we load your content...