Self-assembly of heterometallic LnIII–CoII coordination polymers: syntheses, structures, and magnetic studies†
Abstract
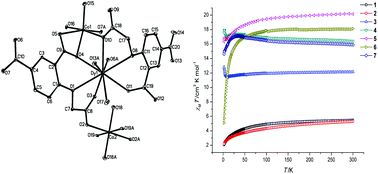
A family of heterometallic 4f–3d coordination polymers with the common formula [LnCo1.5(L)2(H2O)5]n (Ln = Pr(1), Eu(2), Gd(3), Tb(4), Dy(5), Ho(6), and Er(7), and H3L = 4-(carboxymethoxy)isophthalic acid) was obtained under solvothermal conditions with the effect of NaN3, which was involved in the reactions but not in the final structure. All of the compounds were isostructural, and had a two-dimensional wave-like structure based on centrosymmetric [LnO2Co]–Co–[LnO2Co] motifs. The results of the magnetic measurements displayed that ferromagnetic interactions occurred in the frameworks (3–7) containing “heavy” lanthanide ions (Gd, Tb, Dy, Ho, and Er), which should be ascribed to the coupling between the LnIII and CoII ions. Furthermore, compound 5, containing DyIII ions, displayed field-induced slow magnetic relaxation.

 Please wait while we load your content...
Please wait while we load your content...