Structure investigations on oxygen fluorides†
Abstract
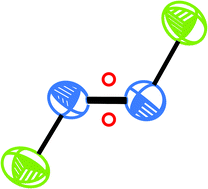
The crystal structure of O2F2 is obtained at −180 °C. In the solid state the molecule has the typical hydrogen peroxide structure that has been established long ago by electron diffraction and microwave spectroscopy. OF2 melts at −223.8 °C, so its structure is determined by powder X-ray data. The structure differs from the solid state structures of ozone and Br2O. O2F in its dissolved form as O2+ HnFn+1− oxidizes palladium to the four valence state, as found some time ago. The first product formed at low temperatures is (O2+H3Pd2F12−)n.
- This article is part of the themed collection: Fluorine

 Please wait while we load your content...
Please wait while we load your content...