A self-assembled octanuclear complex bearing the uncommon close-packed {Fe4Mn4(μ4-O)4(μ-O)4} molecular core†
Abstract
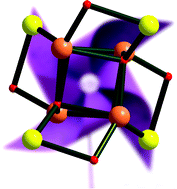
A one-pot open-air reaction of manganese powder with iron(II) chloride in DMF solution of the Schiff base (H2L) formed in situ from salicylaldehyde and hydroxylamine hydrochloride yields the heterometallic complex [Fe4(μ4-O)4Mn4(L)8(DMF)4]·2DMF (1). Single crystal X-ray analysis shows that its molecular structure is based on the octanuclear core {Fe4Mn4(μ4-O)4(μ-O)4} with a quite rare molecular structure type {M8(μ4-X)4(μ-X)4}, where the central cube-like iron motif is modified with four terminal manganese fragments, the whole core being presented as the {Fe4(μ4-O)4} + 4{Mn(μ-O)} combination. Using the data from the Cambridge Structural Database (CSD), an analysis of the octanuclear structures with similar central {M4(μ4-X)4} fragments was performed. The hierarchical order of molecular structure types with the general formula M8Xn for such compounds was proposed and the topological features as well as the factors that influence the molecular type formation are discussed. Variable-temperature (1.8–300 K) magnetic susceptibility measurements reveal an antiferromagnetic coupling among the magnetic centres in 1.


 Please wait while we load your content...
Please wait while we load your content...