Cyclodextrin-based PNN supramolecular assemblies: a new class of pincer-type ligands for aqueous organometallic catalysis†
Abstract
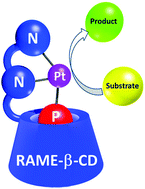
Water-soluble cyclodextrins (CDs) bearing two nitrogen atoms as metal coordinating sites have been synthesized. An appropriate phosphane could be included within their cavity through the primary face to form self-assembled PNN supramolecular edifices. Once the PNN ligands were coordinated to platinum, the resulting complexes proved to be very effective as catalysts in a domino reaction, where a Pt-catalyzed reduction of nitrobenzene was followed by a Paal–Knorr pyrrole reaction. In the nitrobenzene reduction, the modified CDs acted both as first- and second-sphere ligands. Contrary to an acyclic glucopyranose-based NN ligand unable to interact with a phosphane ligand, the CD-based PNN ligands stabilized the catalytic species in water by supramolecular means. Interestingly, the product and the water-soluble Pt-catalyst could be recovered in two different phases once the reaction was complete.

 Please wait while we load your content...
Please wait while we load your content...