Photoswitching a molecular catalyst to regulate CO2 hydrogenation†
Abstract
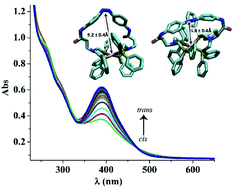
Inspired by nature's ability to regulate catalysis using physiological stimuli, azobenzene was incorporated into Rh(bis)diphosphine CO2 hydrogenation catalysts to photoinitiate structural changes to modulate the resulting catalytic activity. The rhodium bound diphosphine ligands (P(Ph2)-CH2-N(R)-CH2-P(Ph2)) contain the terminal amine of a non-natural amino acid, with the R-group being either β-alanine (β-Ala) or γ-aminobutyric acid (GABA). For both β-Ala and GABA containing complexes, the carboxylic acids of the amino acids were coupled to the amines of diaminoazobenzene, creating a complex consisting of a rhodium bound to a photo-responsive tetradentate ligand. The photo-induced cis–trans isomerization of the azobenzene-containing complexes imposes structural changes on these complexes, as evidenced by NMR studies. We found that the CO2 hydrogenation activity for the β-Ala bound rhodium complex is 40% faster at 27 °C with the light on, i.e. azobenzene in the cis-conformation (TOF = 16 s−1) than when the complex was in the dark and the azobenzene in the trans-conformation (TOF = 11 s−1). In contrast the γ-aminobutyric acid containing rhodium complex has the same rate (TOF ∼17 s−1) with the azobenzene in either the cis or the trans-conformation at 27 °C. The corresponding (bis)diphosphine complexes without the attached azobenzene were also prepared, characterized, and catalytically tested for comparison, and have TOF's of 30 s−1. Computational studies were undertaken to evaluate if the difference in rate between the cis- and trans-azobenzene isomers for the β-Ala bound rhodium complex were due to structural differences. These computational investigations revealed major structural changes between all cis- and trans-azobenzene structures, but only minor structural changes that would be unique to the β-Ala bound rhodium complex. We postulate that the different rates between the cis- and trans-azobenzene β-Ala bound containing rhodium complexes are due to subtle changes in the bite angle arising from steric strain due to the azobenzene-containing tetradentate ligand. This strain alters the hydricity of the subsequent rhodium hydride and consequently the rate.

 Please wait while we load your content...
Please wait while we load your content...