Characterization of a Rh(iii) porphyrin–CO complex: its structure and reactivity with an electron acceptor†
Abstract
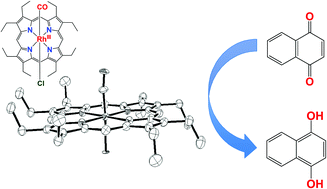
To analyse the electrocatalytic oxidation of carbon monoxide by Rh porphyrins, we isolated a CO-adduct of Rh octaethylporphyrin, and examined its properties and reactivity by IR, NMR, and X-ray crystallographic analyses. The results indicate that the CO adduct of Rh octaethylporphyrin is vulnerable to nucleophilic attack by H2O. The CO-adduct was easily oxidized by an electron acceptor (1,4-naphthoquinone) to generate CO2. This indicates that CO is sufficiently activated in the CO complex of Rh octaethylporphyrin to reduce an electron acceptor. This mechanism is in contrast to that for the CO oxidation by Pt-based electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...