Synthesis of a series of new platinum organometallic complexes derived from bidentate Schiff-base ligands and their catalytic activity in the hydrosilylation and dehydrosilylation of styrene†
Abstract
The synthesis and properties of a novel class of platinum complexes containing Schiff bases as O,N-bidentate ligands is described as are the solution and solid state properties of the uncomplexed ligands. The platinum complexes were prepared from [PtBr2(COD)] (COD = 1,5-cyclooctadiene) and N-(2-hydroxy-1-naphthalidene)aniline derivatives in the presence of base (NaOBut). Instead of a substitution reaction to afford cationic species, the addition of the Schiff base ligands results in both the formal loss of two equivalents of bromide and addition of hydroxide to the COD ligand of the complexes. It is proposed that this reaction proceeds through a cationic platinum complex [Pt(N–O)(COD)]Br which then undergoes addition of water and loss of HBr. An example of a dinuclear platinum complex in which two cyclo–octene ligands are bridged by an ether linkage is also reported. The platinum complexes were evaluated as catalysts for the hydrogenative and dehydrogenative silylation of styrene, the resulting behaviour is substituent, time and temperature dependent.
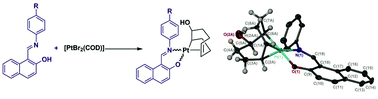

 Please wait while we load your content...
Please wait while we load your content...