A chromium precursor for the Phillips ethylene trimerization catalyst: (2-ethylhexanoate)2CrOH†
Abstract
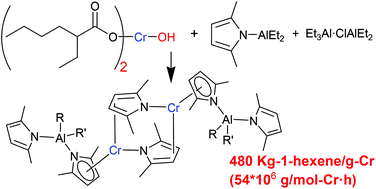
The conventional Phillips ethylene trimerization catalyst prepared by reacting Cr(EH)3 (EH = 2-ethylhexanoate), 2,5-dimethylpyrrole (Me2C4H2NH), Et3Al, and Et2AlCl in an aromatic hydrocarbon solvent was improved to obtain a congener composed of a new chromium precursor (EH)2CrOH, (Me2C4H2N)AlEt2, and Et3Al·ClAlEt2. Reaction of CrCl3 with 3 equiv. Na(EH) in water did not generate Cr(EH)3, but unexpectedly produced (EH)2CrOH. In comparison with the erratic catalytic performance of the original Phillips system, due to the ill-defined nature of the Cr(EH)3 source (16 or 6.8 × 106 g per mol-Cr h depending on the source), the improved system exhibited consistently high activity (54 × 106 g per mol-Cr h). Reaction of (EH)2CrOH with (Me2C4H2N)AlMe2·OEt2 afforded the dimeric Cr(II)-complex (6) coordinated by (η5-Me2C4H2N)AlMe2(NC4H2Me2) and μ2–κ1:η2-Me2C4H2N ligands. 6 provided highly active species when activated with Et3Al·ClAlEt2.

 Please wait while we load your content...
Please wait while we load your content...