Acetate anion-triggered peroxygenation of non-native substrates by wild-type cytochrome P450s†
Abstract
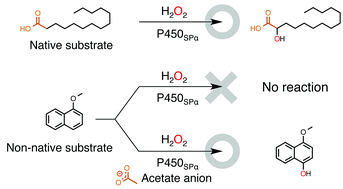
Cytochrome P450SPα (P450SPα) and cytochrome P450BSβ (P450BSβ) belonging to the CYP152 family of enzymes (CYP152s) can utilize H2O2 efficiently as an oxidant for the generation of compound I. Although P450SPα and P450BSβ have very high substrate specificity and catalyse hydroxylation of long-chain fatty acids exclusively, we found that they can oxidize non-native substrates such as styrene simply by including medium chain length n-alkyl carboxylic acids as “decoy molecules.” Although we had assumed that acetic acid did not serve as a decoy molecule, P450SPα and P450BSβ efficiently catalysed oxidation of non-native substrates when the reaction was carried out at a high concentration of acetate anion. The turnover rate for epoxidation of styrene catalysed by P450BSβ in the presence of 1 M acetate anion reached 590 ± 30 min−1.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...