A study on the thermal conversion of scheelite-type ABO4 into perovskite-type AB(O,N)3†
Abstract
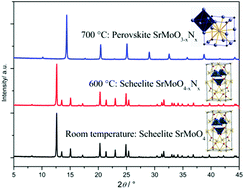
Phase-pure scheelite AMoO4 and AWO4 (A = Ba, Sr, Ca) were thermally treated under an ammonia atmosphere at 400 to 900 °C. SrMoO4 and SrWO4 were shown to convert into cubic perovskite SrMoO2N and SrWO1.5N1.5, at 700 °C and 900 °C respectively, and to form metastable intermediate phases (scheelite SrMoO4−xNx and SrWO4−xNx), as revealed by X-ray diffraction (XRD), elemental analysis and FTIR spectroscopy. High-temperature oxide melt solution calorimetry reveals that the enthalpy of formation for SrM(O,N)3 (M = Mo, W) perovskites is less negative than that of the corresponding scheelite oxides, though the conversion of the scheelite oxides into perovskite oxynitrides is thermodynamically favorable at moderate temperatures. The reaction of BaMO4 with ammonia leads to the formation of rhombohedral Ba3M2(O,N)8 and the corresponding binary metal nitrides Mo3N2 and W4.6N4; similar behavior was observed for CaMO4, which converted upon ammonolysis into individual oxides and nitrides. Thus, BaMO4 and CaMO4 were shown to not provide access to perovskite oxynitrides. The influence of the starting scheelite oxide precursor, the structure distortion and the degree of covalency of the B-site-N bond are discussed within the context of the formability of perovskite oxynitrides.

 Please wait while we load your content...
Please wait while we load your content...