A tricarboxylated PtCl(terpyridine) derivative exhibiting pH-dependent photocatalytic activity for H2 evolution from water†
Abstract
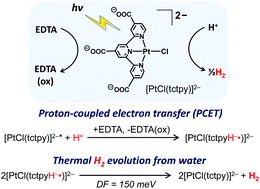
The first negatively charged PtCl(tpy) (tpy = 2,2′:6′,2′′-terpyridine) derivative, formulated as Na2[PtCl(tctpy)]·5H2O (tctpy = 2,2′:6′,2′′-terpyridine-4,4′,4′′-tricarboxylate), was prepared, characterized, and investigated in detail for its activity as a single-component photocatalyst that drives water reduction to H2 in the presence of a sacrificial electron donor (EDTA). This compound was confirmed to exist in its fully deprotonated form [PtCl(tctpy)]2− in aqueous media at pH > 4.4. Despite its dianionic character, [PtCl(tctpy)]2− was found to form a specific adduct with anionic EDTA (i.e., YH22− and YH3−, where YH4 is a fully protonated form of EDTA), enabling reductive quenching of the triplet metal-to-ligand charge transfer excited state within the adduct, leading to subsequent electron transfer steps correlated with Pt(II)-catalyzed H2 evolution from water. Electrochemical studies also reveal that the compound exhibits a unique pH-dependent first reduction (i.e., tctpy-centered reduction), leading to our realization of the first example of a Pt(II)-based molecular system that photocatalyzes the H2 evolution reaction accompanied by a ligand-based proton-coupled electron transfer (PCET) process.

 Please wait while we load your content...
Please wait while we load your content...