Exceptional sensitivity to the synthetic approach and halogen substituent for Zn(ii) coordination assemblies with 5-halonicotinic acids†
Abstract
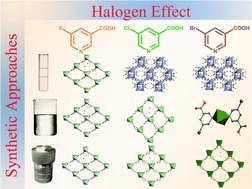
Seven Zn(II) coordination complexes with 5-halonicotinic acids (HL-X, X = F, Cl, or Br) have been synthesized with different synthetic approaches, including layer diffusion or stirring method in an ambient environment and solvothermal synthesis at 100 °C. Assembly of HL-F with Zn(II) under different conditions will yield the same 2D network of [Zn(L-F)2]n (1). Interestingly, three distinct complexes, a 3D framework {[Zn2(L-Cl)4(H2O)](H2O)6}n (2) and two 2D pseudo-polymorphic isomers {[Zn(L-Cl)2](H2O)1.5}n (3) and {[Zn2(L-Cl)4](H2O)}n (4) can be obtained by reacting HL-Cl with Zn(II) under layer diffusion, stirring, and solvothermal conditions, respectively. Furthermore, replacing the –Cl substituent with –Br on the HL-X ligand will also afford three diverse coordination assemblies of 3D {[Zn2(L-Br)4(H2O)](CH3OH)2.5}n (5), mononuclear [Zn(HL-Br)2(H2O)4][L-Br]2 (6), and 2D {[Zn(L-Br)2](H2O)1.15}n (7) depending on the synthetic pathways. Beyond the significant influence of the synthetic approach, which will lead to the formation of various crystalline products, the halogen substitution effect of HL-X ligands on the coordination motifs has also been demonstrated. In addition, thermal stability and fluorescence for these crystalline materials will be presented.


 Please wait while we load your content...
Please wait while we load your content...