An experimental and theoretical magneto-structural study of polynuclear NiII complexes assembled from a versatile bis(salicylaldehyde)diamine polytopic ligand†
Abstract
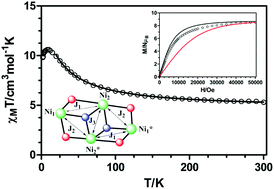
Six novel NiII complexes, ranging from mononuclear to tetranuclear, have been prepared from the polytopic symmetrical Mannich base ligand N,N′-dimethyl-N,N′-bis(2-hidroxy-3-formyl-5-bromo-benzyl)ethylenediamine (H2L) and different anionic coligands: [Ni(H2L)(NO3)(H2O)]NO3·H2O (1), [Ni2(μ-L)(acac)2(H2O)]·CH3CN (2), [Ni2(μ-L)(μ-OAc)(NCS)] (3), [Ni3(μ-L)2(μ-OH2)2(H2O)(CH3CN)](NO3)2·4CH3CN (4), [Ni4(μ-L)2(μ-OAc)2(μ-OCH3)2]·6H2O·2CH3OH (5) and [Ni4(μ-L)2(μ-OAc)2(μ-N3)2]·2H2O·CH3OH (6). These complexes have been characterized by single crystal X-ray diffraction, magnetic measurements and DFT theoretical calculations. The structural analysis of these complexes reveals that the anionic coligand and reaction conditions play a fundamental role in determining their final structures and magnetic properties. Compound 1 contains a monomeric cationic unit with the nickel ion coordinated in the external O4 site of the compartmental ligand H2L, which acts in a neutral zwitterionic form. Complexes 2 and 3 are dinuclear Ni2 neutral entities, in which the NiII ions are connected through two μ-phenoxido bridging groups. The Ni(O)2Ni bridging fragment in 2 is almost planar, whereas in 3 is bent due to the additional presence of a syn–syn acetate bridge connecting the NiII atoms. Complex 4 has a bent trinuclear structure with double μ-phenoxido/μ-water bridges between the central and terminal nickel atoms. Complexes 5 and 6 are Ni4 complexes with defective dicubane structures, in which triple μ-phenoxido/μ1,1,1-X/syn–syn acetate and double μ-phenoxido/μ1,1,1-X mixed bridges connect central and terminal NiII atoms, whereas double μ1,1,1-X bridging ligands link the central NiII ions (X = methoxido and azido groups for 5 and 6, respectively). Magnetic susceptibility measurements reveal that complex 2 shows a moderate antiferromagnetic interaction between the NiII ions through the double di-μ-phenoxido bridge, leading to a S = 0 ground state. Compared to 2, complex 3 shows a much weaker magnetic exchange interaction due the counter-complementarity effect provoked by the additional presence of the syn–syn acetate bridging group, as well as the non-planarity of the bridging fragment. In complex 4, the double μ-phenoxido/μ-water mixed bridges lead to very weak antiferromagnetic interactions between the central and external NiII ions. Overall ferromagnetic interactions are found for 3, 5 and 6, although in 5 not all the magnetic pathways transmit ferromagnetic interactions. A detailed analysis of the magnetic exchange interactions transmitted through the different pathways as well as DFT calculations on the X-ray structures of compounds 2–6 were performed to support the magneto-structural data of these compounds.

 Please wait while we load your content...
Please wait while we load your content...