Selective turn-off phosphorescent and colorimetric detection of mercury(ii) in water by half-lantern platinum(ii) complexes†
Abstract
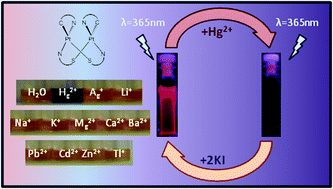
The platinum(II) half-lantern dinuclear complexes [{Pt(bzq)(μ-C7H4NS2-κN,S)}2] (A) and [{Pt(bzq)(μ-C7H4NOS-κN,S)}2] (B) [bzq = benzo[h]quinolinate, C7H4NS2 = 2-mercaptobenzothiazolate, C7H4NOS = 2-mercaptobenzoxazolate] in solution of DMSO–H2O undergo a dramatic color change from yellowish-orange to purple and turn-off phosphorescence in the presence of a small amount of Hg2+, being discernible by the naked-eye and by spectroscopic methods. Other metal ions as Ag+, Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Pb2+, Cd2+, Zn2+ and Tl+ were tested and, even in a big excess, showed no interference in the selective detection of Hg2+ in water. Job's plot analysis indicated a 1 : 1 stoichiometry in the complexation mode of Hg2+ by A/B. The phosphorescence quenching attributed to the formation of [A/B : Hg2+] complexes showed binding constants of K = 1.13 × 105 M−1 (A) and K = 1.99 × 104 M−1 (B). The limit of detection has been also evaluated. In addition, dried paper test strips impregnated in DMSO solutions of A and B can detect concentration of Hg2+ in water as low as 1 × 10−5 M for B and 5 × 10−5 M for A, making these complexes good candidates to be used as real-time Hg2+ detectors. The nature of the interaction of the Pt2 half-lantern complex A with the Hg2+ cation, has been investigated by theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...