Manganese borohydride; synthesis and characterization†
Abstract
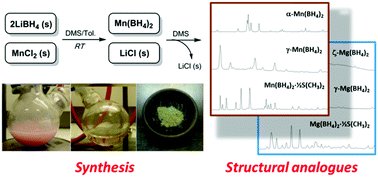
Solvent-based synthesis and characterization of α-Mn(BH4)2 and a new nanoporous polymorph of manganese borohydride, γ-Mn(BH4)2, via a new solvate precursor, Mn(BH4)2·1/2S(CH3)2, is presented. Manganese chloride is reacted with lithium borohydride in a toluene/dimethylsulfide mixture at room temperature, which yields halide and solvent-free manganese borohydride after extraction with dimethylsulfide (DMS) and subsequent removal of residual solvent. This work constitutes the first example of establishing a successful, reproducible solvent-based synthesis route for a pure, crystalline, stable transition metal borohydride. The new polymorph, γ-Mn(BH4)2, is shown to be the manganese counterpart of the zeolite-like compound, γ-Mg(BH4)2 (cubic, a = 16.209(1) Å, space group Id![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) a). It is verified that large pores (diameter > 6.0 Å) exist in this structure. The solvate, Mn(BH4)2·1/2S(CH3)2, is subsequently shown to be the analogue of Mg(BH4)2·1/2S(CH3)2. As the structural analogies between Mg(BH4)2 and Mn(BH4)2 became evident a new polymorph of Mg(BH4)2 was identified and termed ζ-Mg(BH4)2. ζ-Mg(BH4)2 is the structural counterpart of α-Mn(BH4)2. All synthesis products are characterized employing synchrotron radiation-powder X-ray diffraction, infrared spectroscopy and thermogravimetric analysis in combination with mass spectroscopy. Thermal analysis reveals the decomposition of Mn(BH4)2 to occur at 160 °C, accompanied by a mass loss of 14.8 wt%. A small quantity of the desorbed gaseous species is identified as diborane (ρm(Mn(BH4)2) = 9.5 wt% H2), while the remaining majority is found to be hydrogen.
a). It is verified that large pores (diameter > 6.0 Å) exist in this structure. The solvate, Mn(BH4)2·1/2S(CH3)2, is subsequently shown to be the analogue of Mg(BH4)2·1/2S(CH3)2. As the structural analogies between Mg(BH4)2 and Mn(BH4)2 became evident a new polymorph of Mg(BH4)2 was identified and termed ζ-Mg(BH4)2. ζ-Mg(BH4)2 is the structural counterpart of α-Mn(BH4)2. All synthesis products are characterized employing synchrotron radiation-powder X-ray diffraction, infrared spectroscopy and thermogravimetric analysis in combination with mass spectroscopy. Thermal analysis reveals the decomposition of Mn(BH4)2 to occur at 160 °C, accompanied by a mass loss of 14.8 wt%. A small quantity of the desorbed gaseous species is identified as diborane (ρm(Mn(BH4)2) = 9.5 wt% H2), while the remaining majority is found to be hydrogen.

 Please wait while we load your content...
Please wait while we load your content...