The structure, magnetism and EPR spectra of a (μ-thiophenolato)(μ-pyrazolato-N,N′) double bridged dicopper(ii) complex†
Abstract
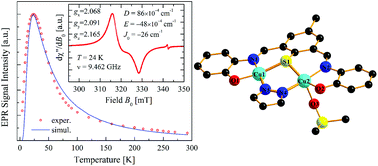
A new binuclear copper(II) complex, namely [Cu2L(pz)(DMSO)], where L = 2,6-bis[(2-phenoxy)iminomethyl]-4-methylthiophenolate(3-) and pz = pyrazolate ligand, has been synthesized by a one-pot synthesis involving copper(II) acetate monohydrate, the S-protected ligand precursor 2-(N,N-dimethylthiocarbamato)-5-methylisophthalaldehyde di-2′-hydroxy anil, (I), and pyrazole, in which a metal-promoted S-deprotection reaction occurs during the formation of the complex. This was characterized by routine physicochemical studies, single crystal X-ray diffraction and electron paramagnetic resonance (EPR) techniques. The structure analysis reveals that there are copper centres in two different environments, a slightly distorted square planar and a distorted square-pyramidal, arranged in binuclear units. The EPR study of these binuclear units performed at 9.4 GHz in the temperature range between 4 and 293 K shows an antiferromagnetic interaction between CuII ions, and allows evaluating g factors gx = 2.068(1), gy = 2.091(1) and gz = 2.165(1), with <g> = 2.108(1), an exchange coupling parameter J0 = −26(1) cm−1 (defined as 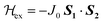 ), and a zero field splitting of the ground triplet state described by D = 86(2) × 10−4 cm−1 and E = −48(3) × 10−4 cm−1. These results are discussed and compared with the existing literature.
), and a zero field splitting of the ground triplet state described by D = 86(2) × 10−4 cm−1 and E = −48(3) × 10−4 cm−1. These results are discussed and compared with the existing literature.


 Please wait while we load your content...
Please wait while we load your content...