Vanadyl cationic complexes as catalysts in olefin oxidation†
Abstract
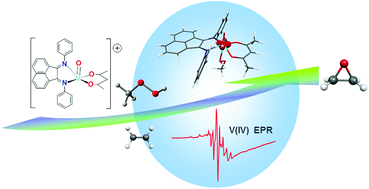
Three new mononuclear oxovanadium(IV) complexes [VO(acac)(R-BIAN)]Cl (BIAN = 1,2-bis{(R-phenyl)imino}acenaphthene, R = H, 1; CH3, 2; Cl, 3) were prepared and characterized. They promoted the catalytic oxidation of olefins such as cyclohexene, cis-cyclooctene, and styrene with both tbhp (tert-butylhydroperoxide) and H2O2, and of enantiopure olefins (S(−)- and R(+)-pinene, and S(−)- and R(+)-limonene) selectively to their epoxides, with tbhp as the oxidant. The TOFs for styrene epoxidation promoted by complex 3 with H2O2 (290 mol mol−1V h−1) and for cis-cyclooctene epoxidation by 2 with tbhp (248 mol mol−1V h−1) are particularly good. Conversions reached 90% for several systems with tbhp, and were lower with H2O2. A preference for the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, rather than the terminal one, was found for limonene. Kinetic data indicate an associative process as the first step of the reaction and complex [VO(acac)(H-BIAN)]+ (1+) was isolated in an FTICR cell after adding tbhp to 1. EPR studies provide evidence for the presence of a V(IV) species in solution, until at least 48 hours after the addition of tbhp and cis-cyclooctene, and cyclic voltammetry studies revealed an oxidation potential above 1 V for complex 1. DFT calculations suggest that a [VO(H-BIAN)(MeOO)]+ complex is the likely active V(IV) species in the catalytic cycle from which two competitive mechanisms for the reaction proceed, an outer sphere path with an external attack of the olefin at the coordinated peroxide, and an inner sphere mechanism starting with a complex with the olefin coordinated to vanadium.
C bond, rather than the terminal one, was found for limonene. Kinetic data indicate an associative process as the first step of the reaction and complex [VO(acac)(H-BIAN)]+ (1+) was isolated in an FTICR cell after adding tbhp to 1. EPR studies provide evidence for the presence of a V(IV) species in solution, until at least 48 hours after the addition of tbhp and cis-cyclooctene, and cyclic voltammetry studies revealed an oxidation potential above 1 V for complex 1. DFT calculations suggest that a [VO(H-BIAN)(MeOO)]+ complex is the likely active V(IV) species in the catalytic cycle from which two competitive mechanisms for the reaction proceed, an outer sphere path with an external attack of the olefin at the coordinated peroxide, and an inner sphere mechanism starting with a complex with the olefin coordinated to vanadium.


 Please wait while we load your content...
Please wait while we load your content...