New AMD3100 derivatives for CXCR4 chemokine receptor targeted molecular imaging studies: synthesis, anti-HIV-1 evaluation and binding affinities†
Abstract
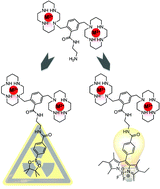
CXCR4 is a target of growing interest for the development of new therapeutic drugs and imaging agents as its role in multiple disease states has been demonstrated. AMD3100, a CXCR4 chemokine receptor antagonist that is in current clinical use as a haematopoietic stem cell mobilising drug, has been widely studied for its anti-HIV properties, potential to inhibit metastatic spread of certain cancers and, more recently, its ability to chelate radiometals for nuclear imaging. In this study, AMD3100 is functionalised on the phenyl moiety to investigate the influence of the structural modification on the anti-HIV-1 properties and receptor affinity in competition with anti-CXCR4 monoclonal antibodies and the natural ligand for CXCR4, CXCL12. The effect of complexation of nickel(II) in the cyclam cavities has been investigated. Two amino derivatives were obtained and are suitable intermediates for conjugation reactions to obtain CXCR4 molecular imaging agents. A fluorescent probe (BODIPY) and a precursor for 18F (positron emitting isotope) radiolabelling were conjugated to validate this route to new CXCR4 imaging agents.
- This article is part of the themed collection: Dalton Discussion 15: Metal ions in medical imaging

 Please wait while we load your content...
Please wait while we load your content...