N-heterocyclic carbene gold(i) and silver(i) complexes bearing functional groups for bio-conjugation†
Abstract
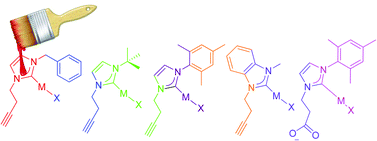
This work describes several synthetic approaches to append organic functional groups to gold and silver N-heterocyclic carbene (NHC) complexes suitable for applications in biomolecule conjugation. Carboxylate appended NHC ligands (3) lead to unstable AuI complexes that convert into bis-NHC species (4). A benzyl protected carboxylate NHC–AuI complex 2 was synthesized but deprotection to produce the carboxylic acid functionality could not be achieved. A small library of new alkyne functionalized NHC proligands were synthesized and used for subsequent silver and gold metalation reactions. The alkyne appended NHC gold complex 13 readily reacts with benzyl azide in a copper catalyzed azide–alkyne cycloaddition reaction to form the triazole appended NHC gold complex 14. Cell cytotoxicity studies were performed on DLD-1 (colorectal adenocarcinoma), Hep-G2 (hepatocellular carcinoma), MCF-7 (breast adenocarcinoma), CCRF-CEM (human T-Cell leukemia), and HEK (human embryonic kidney). Complete spectroscopic characterization of the ligands and complexes was achieved using 1H and 13C NMR, gHMBC, ESI-MS, and combustion analysis.

 Please wait while we load your content...
Please wait while we load your content...