Synthesis and coordination chemistry of 1,1,1-tris-(pyrid-2-yl)ethane†
Abstract
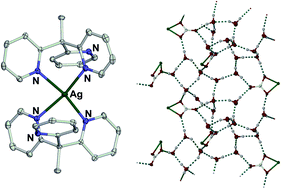
A new synthesis of 1,1,1-tris(pyrid-2-yl)ethane (L), and a survey of its coordination chemistry, are reported. The complexes [ML2]n+ (Mn+ = Fe2+, Co2+, Co3+, Cu2+ and Ag+), [PdCl2L] and [CuI(L)] have all been crystallographically characterised. Noteworthy results include an unusual square planar silver(I) complex [Ag(L)2]X (X− = NO3− and SbF6−); the oxidative fixation of aerobic CO2 by [CuI(L)] to yield [Cu2I(L)2(μ-CO3)]2[CuI3] and [Cu(CO3)(L)]; and, water/carbonato tape and water/iodo layer hydrogen bonding networks in hydrate crystals of two of the copper(II) complexes. Cyclic voltammetric data on [Fe(L)2]2+ and [Co(L)2]2+/3+ imply that the peripheral methyl substituent has a weak influence on the ligand field exerted by L onto a coordinated metal ion.

 Please wait while we load your content...
Please wait while we load your content...