Abnormal luminescent property of Mn2+ in α-LiZnBO3:Mn2+
Abstract
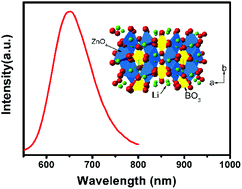
Mn2+-activated red phosphor α-LiZnBO3:Mn2+ was synthesized by solid state reaction. ESR spectra prove that the doped ions are Mn2+. The doped Mn2+ ion is inclined to occupy Zn2+ site, which is a tetrahedral coordination. The diffuse reflection spectra indicate that α-LiZnBO3:Mn2+ has strong absorption in the range of 400–450 nm. Excited at 431 nm, an abnormal red emission band in the wavelength of 550–800 nm is observed, which is because of the strong crystal field induced by the distorted tetrahedral. The emission bands are centered at 647 nm, regardless of the excitation wavelength and Mn2+ doping concentration. The temperature-dependent PL results reveal that α-LiZnBO3:Mn2+ is thermally stable but the emission peak moves to shorter wavelength as temperature increases because of the decrease of the crystal field.

 Please wait while we load your content...
Please wait while we load your content...