The influence of catalyst amount and Pd loading on the H2O2 synthesis from hydrogen and oxygen†
Abstract
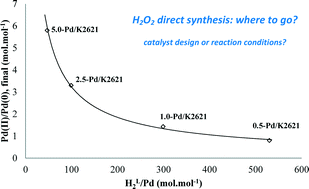
Palladium catalysts with an active metal content from 0.3 to 5.0 wt.% and supported on a strongly acidic, macroporous resin were prepared by ion-exchange/reduction method. H2O2 direct synthesis was carried out in the absence of promoters (acids and halides). The total Pd amount in the reacting environment was varied by changing A) the catalyst concentration in the slurry and B) the Pd content of the catalyst. In both cases, smaller amounts of the active metal enhance the selectivity towards H2O2, at any H2 conversion, with option B) better than A). In case A), the Pd(II)/Pd(0) molar ratio (XPS) in the spent catalysts was found to decrease at lower catalyst Pd content. With these catalysts and this experimental set-up the dynamic HL2/Pd molar ratio, the metal loading and the metal particle size were the key factors controlling the selectivity, which reached 57% at 60% H2 conversion, and 80% at lower conversion.


 Please wait while we load your content...
Please wait while we load your content...