Variation of redox activity and synergistic effect for improving the preferential oxidation of CO in H2-rich gases in porous Pt/CeO2–Co3O4 catalysts†
Abstract
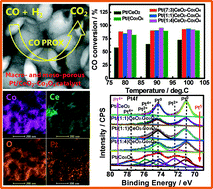
Porous Pt/CeO2–Co3O4 catalysts are fabricated via thermal decomposition of cerium nitrate hexahydrate and cobaltous acetate tetrahydrate precursors using polystyrene sphere colloidal crystals as templates followed by Pt impregnation. The obtained catalysts possess well-defined macroporous skeletons and mesoporous walls, adjustable chemical compositions, and varied surface elemental states. The macro- and meso-porous Pt/CeO2–Co3O4 catalysts show superior catalytic performance to traditional powder Pt/CeO2–Co3O4 catalysts for CO preferential oxidation in H2-rich gases. The redox activity and the valence state of the macro- and meso-porous Pt/CeO2–Co3O4 catalysts are largely varied depending on their chemical compositions and further cause the strong synergy between Pt, CeO2 and Co3O4, which accounts for the improvement of catalytic performance. Meanwhile, the macro- and meso-porous structure in Pt/CeO2–Co3O4 catalysts is conducive to the diffusion of reactants and products, thus enhancing the catalytic performance simultaneously.

 Please wait while we load your content...
Please wait while we load your content...