Simple soluble Bi(iii) salts as efficient catalysts for the oxidation of alkanes with H2O2†
Abstract
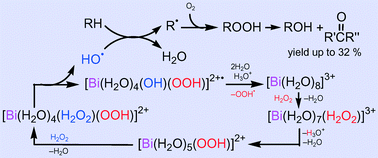
A simple catalytic system based on a soluble bismuth(III) salt, Bi(NO3)3/H2O2/HNO3/CH3CN + H2O, exhibits pronounced activity towards the homogeneous oxidation of inert alkanes with the yield of oxygenate products up to 32% and TON up to 112. The experimental selectivity parameters and kinetic data together with theoretical DFT calculations indicate that the reaction occurs via a free radical mechanism involving the formation of the HO˙ radicals which directly react with alkane molecules. The mechanism of the HO˙ generation (which is the rate limiting step of the whole process) includes the substitution of a water ligand for H2O2 in the initial aqua complex [Bi(H2O)8]3+, hydrolysis of the coordinated H2O2, second H2O-for-H2O2 substitution and the homolytic HO–OH bond cleavage in complex [Bi(H2O)4(H2O2)(OOH)]2+ (6). The relatively low overall activation energy for this process (ca. 20 kcal mol−1) is accounted for by the high lability and acidity of the Bi aqua complexes and tremendous activation of coordinated H2O2 in 6 towards homolysis.

 Please wait while we load your content...
Please wait while we load your content...