The reactivity of the active metal oxo and hydroxo intermediates and their implications in oxidations
Abstract
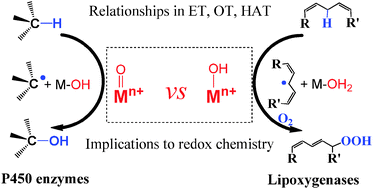
While the significance of the redox metal oxo moieties has been fully acknowledged in versatile oxidation processes, active metal hydroxo moieties are gradually realized to play the key roles in certain biological oxidation events, and their reactivity has also been evidenced by related biomimic models. However, compared with the metal oxo moieties, the significance of the metal hydroxo moieties has not been fully recognized, and their relationships in oxidations remain elusive until recently. This review summarizes the reactivity of the metal oxo and hydroxo moieties in different oxidation processes including hydrogen atom transfer, oxygen atom transfer and electron transfer, and their reactivity similarities and differences have been discussed as well. Particularly, how the physicochemical properties like metal–oxygen bond order, net charge and potential of a redox metal ion affect its reactivity has also been presented based on available data. We hope that this review may provide new clues to understand the origins of the enzyme's choice on them in a specific event, to explain the elusive phenomena occurring in those enzymatic, homogeneous and heterogeneous oxidations, to design selective redox catalysts and control their reactivity.

 Please wait while we load your content...
Please wait while we load your content...