Acid–base equilibrium dynamics in methanol and dimethyl sulfoxide probed by two-dimensional infrared spectroscopy†
Abstract
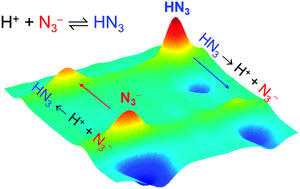
Two-dimensional infrared (2DIR) spectroscopy, which has been proven to be an excellent experimental method for studying thermally-driven chemical processes, was successfully used to investigate the acid dissociation equilibrium of HN3 in methanol (CH3OH) and dimethyl sulfoxide (DMSO) for the first time. Our 2DIR experimental results indicate that the acid–base equilibrium occurs on picosecond timescales in CH3OH but that it occurs on much longer timescales in DMSO. Our results imply that the different timescales of the acid–base equilibrium originate from different proton transfer mechanisms between the acidic (HN3) and basic (N3−) species in CH3OH and DMSO. In CH3OH, the acid–base equilibrium is assisted by the surrounding CH3OH molecules which can directly donate H+ to N3− and accept H+ from HN3 and the proton migrates through the hydrogen-bonded chain of CH3OH. On the other hand, the acid–base equilibrium in DMSO occurs through the mutual diffusion of HN3 and N3− or direct proton transfer. Our 2DIR experimental results corroborate different proton transfer mechanisms in the acid–base equilibrium in protic (CH3OH) and aprotic (DMSO) solvents.

 Please wait while we load your content...
Please wait while we load your content...