The cis-isomer performs better than the trans-isomer in porphyrin-sensitized solar cells: interfacial electron transport and charge recombination investigations
Abstract
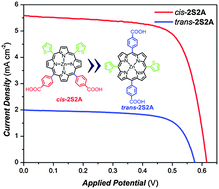
We report characterizations and device performance for dye-sensitized solar cells using cis- and trans-isomers of 2D–π–2A zinc porphyrins with carboxyphenyl and thienyl groups in their meso-positions. Under identical experimental conditions with similar dye loadings, we observed overall power conversion efficiencies of 2.44% and 0.88% for devices made of cis-2S2A and trans-2S2A, respectively. This uneven performance among cis and trans isomers under the same experimental conditions can be rationalized with detailed investigations via spectroscopic, quantum chemical, and femtosecond fluorescence up-conversion investigations. Density functional theory (DFT) calculations show that a small amount of electron density is localized over carboxyphenyl groups in the LUMO of cis-2S2A, but there is no electron density populated on the carboxyphenyl groups in the LUMO of trans-2S2A. The femtosecond fluorescence decay measurements revealed that the excited-state lifetime of trans-2S2A on Al2O3 is half of that of cis-2S2A on Al2O3. Moreover, the dye-to-TiO2 electron injection time of trans-2S2A is 2.54 ps, which is shorter than that of cis-2S2A/TiO2 (2.95 ps). Electrochemical impedance spectra measured under one sun illumination also revealed that the charge recombination time of cis-2S2A is longer than that of trans-2S2A. This thorough understanding of isomeric effects on the performance of porphyrins will serve as a guideline for the design of future sensitizing dyes for solar cells.

 Please wait while we load your content...
Please wait while we load your content...