Bio-inspired design of electrocatalysts for oxalate oxidation: a combined experimental and computational study of Mn–N–C catalysts†
Abstract
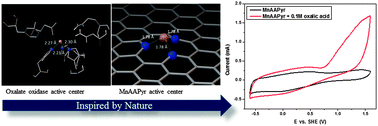
We report a novel non-platinum group metal (non-PGM) catalyst derived from Mn and amino- antipyrine (MnAAPyr) that shows electrochemical activity towards the oxidation of oxalic acid comparable to Pt with an onset potential for oxalate oxidation measured to be 0.714 ± 0.002 V vs. SHE at pH = 4. The material has been synthesized using a templating Sacrificial Support Method with manganese nitrate and 4-aminoantipyrine as precursors. This catalyst is a nano-structured material in which Mn is atomically dispersed on a nitrogen-doped graphene matrix. XPS studies reveal high abundance of pyridinic, Mn–Nx, and pyrrolic nitrogen pointing towards the conclusion that pyridinic nitrogen atoms coordinated to manganese constitute the active centers. Thus, the main features of the MnAAPyr catalyst are it exhibits similarity to the active sites of naturally occurring enzymes that are capable of efficient and selective oxidation of oxalic acid. Density functional theory in plane wave formalism with Perdew, Burke and Ernzerhof functional was further used to study the stability and activity of different one-metal active centers that could exist in the catalyst. The results show that the stability of the Mn–Nx sites changes in the following order: MnN4 > MnN3C > MnN2C2 > MnN3. Based on the overpotentials of 0.64 V and 0.71 V vs. SHE, calculated using the free energy diagrams for the oxalate oxidation mechanism, we could conclude that the MnN3C and MnN2C2 sites are most probable Mn–Nx sites responsible for the reported catalytic activity of the new catalyst.

 Please wait while we load your content...
Please wait while we load your content...