The substituent effect on the MLCT excited state dynamics of Cu(i) complexes studied by femtosecond time-resolved absorption and observation of coherent nuclear wavepacket motion†
Abstract
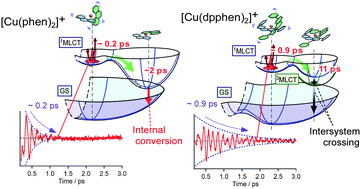
The substituent effect on the excited-state dynamics of bis-diimine Cu(I) complexes was investigated by femtosecond time-resolved absorption spectroscopy with the S1 ← S0 metal-to-ligand charge transfer (MLCT) photoexcitation. The time-resolved absorption of [Cu(phen)2]+ (phen = 1,10-phenanthroline) showed a slight intensity increase of the S1 absorption with a time-constant of 0.1–0.2 ps, reflecting the flattening distortion occurring in the S1 state. The transient absorption of the ‘flattened’ S1 state was clearly observed, although its fluorescence was not observed in the previous fluorescence up-conversion study in the visible region. The flattened S1 state decayed with a time constant of ∼2 ps, and the S0 bleaching recovered accordingly. This clarifies that the S1 state of [Cu(phen)2]+ is predominantly relaxed to the S0 state by internal conversion. The time-resolved absorption of [Cu(dpphen)2]+ (dpphen = 2,9-diphenyl-1,10-phenanthroline) showed a 0.9 ps intensity increase of the S1 absorption due to the flattening distortion, and then exhibited a 11 ps spectral change due to the intersystem crossing. This excited-state dynamics of [Cu(dpphen)2]+ is very similar to that of [Cu(dmphen)2]+ (dmphen = 2,9-dimethyl-1,10-phenanthroline). In the ultrafast pump–probe measurements with 35 fs time resolution, [Cu(phen)2]+ and [Cu(dpphen)2]+ exhibited oscillation due to the nuclear wavepacket motions of the initial S1 state, and the oscillation was damped as the structural change took place. This indicates that the initial S1 states have well-defined vibrational structures and that the vibrational coherence is retained in their short lifetimes. The present time-resolved absorption study, together with the previous time-resolved fluorescence study, provides a unified view for the ultrafast dynamics of the MLCT excited state of the Cu(I) complexes.

 Please wait while we load your content...
Please wait while we load your content...