Rotational dynamics of organic cations in the CH3NH3PbI3 perovskite†
Abstract
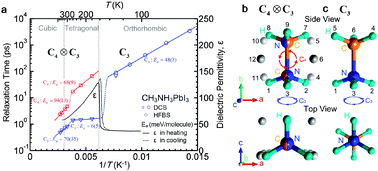
Methylammonium lead iodide (CH3NH3PbI3) based solar cells have shown impressive power conversion efficiencies of above 20%. However, the microscopic mechanism of the high photovoltaic performance is yet to be fully understood. Particularly, the dynamics of CH3NH3+ cations and their impact on relevant processes such as charge recombination and exciton dissociation are still poorly understood. Here, using elastic and quasi-elastic neutron scattering techniques and group theoretical analysis, we studied rotational modes of the CH3NH3+ cation in CH3NH3PbI3. Our results show that, in the cubic (T > 327 K) and tetragonal (165 K < T < 327 K) phases, the CH3NH3+ ions exhibit four-fold rotational symmetry of the C–N axis (C4) along with three-fold rotation around the C–N axis (C3), while in the orthorhombic phase (T < 165 K) only C3 rotation is present. At around room temperature, the characteristic relaxation times for the C4 rotation are found to be τC4 ≈ 5 ps while for the C3 rotation τC3 ≈ 1 ps. The T-dependent rotational relaxation times were fitted with Arrhenius equations to obtain activation energies. Our data show a close correlation between the C4 rotational mode and the temperature dependent dielectric permittivity. Our findings on the rotational dynamics of CH3NH3+ and the associated dipole have important implications for understanding the low exciton binding energy and a slow charge recombination rate in CH3NH3PbI3 which are directly relevant for the high solar cell performance.

 Please wait while we load your content...
Please wait while we load your content...