The atomic and electronic structure of nitrogen- and boron-doped phosphorene
Abstract
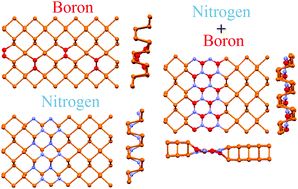
First principles modeling of nitrogen- and boron-doped phosphorene demonstrates the tendency toward the formation of highly ordered structures. Nitrogen doping leads to the formation of –N–P–P–P–N– lines. Further transformation into –P–N–P–N– lines across the chains of phosphorene occurs with increasing band gap and increasing nitrogen concentration, which coincides with the decreasing chemical activity of N-doped phosphorene. In contrast to the case of nitrogen, boron atoms prefer to form –B–B– pairs with the further formation of –P–P–B–B–P–P– patterns along the phosphorene chains. The low concentration of boron dopants converts the phosphorene from a semiconductor into a semimetal with the simultaneous enhancement of its chemical activity. Co-doping of phosphorene by both boron and nitrogen starts from the formation of –B–N– pairs, which provides flat bands and further transformation of these pairs into hexagonal BN lines and ribbons across the phosphorene chains.

 Please wait while we load your content...
Please wait while we load your content...