Synthesis of magnetic mesoporous titania colloidal crystals through evaporation induced self-assembly in emulsion as effective and recyclable photocatalysts†
Abstract
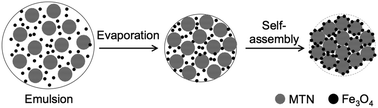
This study illustrates the directed self-assembly of mesoporous TiO2 with magnetic properties due to its colloidal crystal structure with Fe3O4. The Fe3O4 nanoparticles were synthesized using co-precipitation techniques to a size of 28.2 nm and a magnetic saturation of 66.9 emu g−1. Meanwhile, mesoporous titania nanoparticles (MTNs) with a particle diameter of 373 nm, a specific surface area of 236.3 m2 g−1, and a pore size of 2.8 nm were prepared by controlling the rate of hydrolysis. Magnetic colloidal crystals (a diameter of 10.2 μm) were formed by the aggregation of Fe3O4 and MTNs caused by the interface phenomena during solvent evaporation in emulsion. Even the anatase octahedrite produced from the colloidal crystal after a hydrothermal reaction retained a magnetic saturation of 2.8 emu g−1. This study also investigates the photodegradation activity of our synthesized material as a photocatalyst, while utilizing its capability for magnetic separation to prove its usefulness in catalyst recycling.

 Please wait while we load your content...
Please wait while we load your content...