Pressure-dependent formation of i-motif and G-quadruplex DNA structures†
Abstract
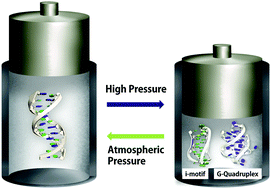
Pressure is an important physical stimulus that can influence the fate of cells by causing structural changes in biomolecules such as DNA. We investigated the effect of high pressure on the folding of duplex, DNA i-motif, and G-quadruplex (G4) structures; the non-canonical structures may be modulators of expression of genes involved in cancer progression. The i-motif structure was stabilized by high pressure, whereas the G4 structure was destabilized. The melting temperature of an intramolecular i-motif formed by 5′-dCGG(CCT)10CGG-3′ increased from 38.8 °C at atmospheric pressure to 61.5 °C at 400 MPa. This effect was also observed in the presence of 40 wt% ethylene glycol, a crowding agent. In the presence of 40 wt% ethylene glycol, the G4 structure was less destabilized than in the absence of the crowding agent. P–T stability diagrams of duplex DNA with a telomeric sequence indicated that the duplex is more stable than G4 and i-motif structures under low pressure, but the i-motif dominates the structural composition under high pressure. Under crowding conditions, the P–T diagrams indicated that the duplex does not form under high pressure, and i-motif and G4 structures dominate. Our findings imply that temperature regulates the formation of the duplex structure, whereas pressure triggers the formation of non-canonical DNA structures like i-motif and G4. These results suggest that pressure impacts the function of nucleic acids by stabilizing non-canonical structures; this may be relevant to deep sea organisms and during evolution under prebiotic conditions.

 Please wait while we load your content...
Please wait while we load your content...