Fluorescence of N-acylated dansylamide with a long hydrophobic tail: sensitive response to premicellar aggregation of sodium deoxycholate†
Abstract
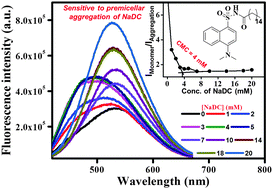
The present work describes the synthesis and photophysical studies of two fluorescent dansylamide derivatives, in which the amine group is acylated by a long hydrophobic chain (a part of a biologically relevant palmitic acid) and by a short hydrophobic tail (a part of acetic acid). The long chain tethered dansyl analogue is successfully utilized in estimating critical micellar concentration (CMC) of bile salts (NaDC, NaC) as well as anionic and cationic surfactants (SDS, CTAB) with the help of enhanced fluorescence intensity facilitated by better solubilization of the molecule in microheterogeneous media. The long chain tethered dansylamide derivative shows significant fluorescence solvatochromism with a red shift (ca. 4000 cm−1) from hexane to water. In contrast, the solvatochromism exhibited by the parent/short acyl chain analogue is much less (ca. 2230 cm−1 from hexane to water) and the fluorescence is not sensitive to micellization. Interestingly, the long chain tethered fluorescent probe shows high sensitivity towards premicellar aggregation of sodium deoxycholate (NaDC) bile salt, through a clear blue shift of emission maxima and concomitant enhancement of fluorescent intensity. Such an observation of fluorescence sensing of premicellar aggregation is unusual.

 Please wait while we load your content...
Please wait while we load your content...