Effects of inorganic acids and divalent hydrated metal cations (Mg2+, Ca2+, Co2+, Ni2+) on γ-AlOOH sol–gel process†
Abstract
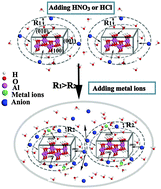
In-depth understanding of the sol–gel process plays an essential role in guiding the preparation of new materials. Herein, the effects of different inorganic acids (HCl, HNO3 and H2SO4) and divalent hydrated metal cations (Mg2+, Ca2+, Co2+, Ni2+) on γ-AlOOH sol–gel process were studied based on experiments and density functional theory (DFT) calculations. In these experiments, the sol originating from the γ-AlOOH suspension was formed only with the addition of HCl and HNO3, but not with H2SO4. Furthermore, the DFT calculations showed that the strong adsorption of HSO4− on the surface of the γ-AlOOH particles, and the hydrogen in HSO4− pointing towards the solvent lead to an unstable configuration of electric double layer (EDL). In the experiment, the gelation time sequence of γ-AlOOH sol obtained by adding metal ions changed when the ionic strength was equal to or greater than 0.198 mol kg−1. The DFT calculations demonstrated that the adsorption energy of hydrated metal ions on the γ-AlOOH surface can actually make a difference in the sol–gel process.

 Please wait while we load your content...
Please wait while we load your content...