The properties of substituted 3D-aromatic neutral carboranes: the potential for σ-hole bonding†
Abstract
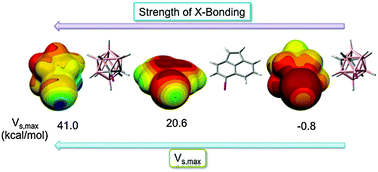
The calculated properties of substituted carboranes such as dipole moment, polarisability, the magnitude of the σ-hole and the desolvation free energy are compared with these properties in comparable aromatic and cyclic aliphatic organic compounds. Dispersion and charge transfer energies are similar. However, the predicted strength of the halogen bonds with the same electron donor (based on the magnitude of the σ-hole) is larger for neutral C-vertex halogen-substituted carboranes than for their organic counterparts. Furthermore, the desolvation penalties of substituted carboranes are smaller than those of the corresponding organic compounds, which should further strengthen the halogen bonds of the former in the solvent. It is predicted that substituted carboranes have the potential to form stronger halogen bonds than comparable aromatic hydrocarbons, which will be even more pronounced in the medium. This theoretical study thus lays ground for the rational engineering of halogen bonding in inorganic crystals as well as in biomolecular complexes.

 Please wait while we load your content...
Please wait while we load your content...