Structural and aggregate analyses of (Li salt + glyme) mixtures: the complex nature of solvate ionic liquids
Abstract
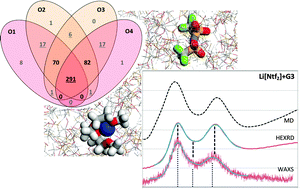
The structure and interactions of different (Li salt + glyme) mixtures, namely equimolar mixtures of lithium bis(trifluoromethylsulfonyl)imide, nitrate or trifluoroacetate salts combined with either triglyme or tetraglyme molecules, are probed using Molecular Dynamics simulations. structure factor functions, calculated from the MD trajectories, confirmed the presence of different amounts of lithium–glyme solvates in the aforementioned systems. The MD results are corroborated by S(q) functions derived from diffraction and scattering data (HEXRD and SAXS/WAXS). The competition between the glyme molecules and the salt anions for the coordination to the lithium cations is quantified by comprehensive aggregate analyses. Lithium–glyme solvates are dominant in the lithium bis(trifluoromethylsulfonyl)imide systems and much less so in systems based on the other two salts. The aggregation studies also emphasize the existence of complex coordination patterns between the different species (cations, anions, glyme molecules) present in the studied fluid media. The analysis of such complex behavior is extended to the conformational landscape of the anions and glyme molecules and to the dynamics (solvate diffusion) of the bis(trifluoromethylsulfonyl)imide plus triglyme system.

 Please wait while we load your content...
Please wait while we load your content...