Mechanical degradation of graphene by epoxidation: insights from first-principles calculations
Abstract
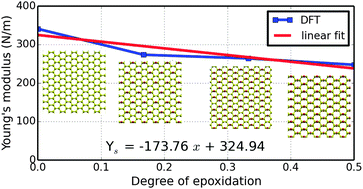
Oxidation is a major cause for the degradation of materials including graphene, where epoxidation (forming the C–O–C bond) is very common. In addition, graphene oxide, in which the epoxy group is one of the two major functional groups (the other is hydroxy), is an important precursor material used for the bulk synthesis of graphene sheets. Information about the mechanical stabilities, non-linear elastic properties, and elastic limits under various strain components is invaluable for application of these nanomaterials. Here, we investigate the mechanical properties of the epoxidized graphene in ordered graphene oxide, namely C6O1, C6O2, and C6O3, representing the carbon : oxygen ratios of 6 : 1, 3 : 1, and 2 : 1, respectively, using first-principles calculations within the framework of density functional theory. We predict a reduction of Young's modulus of graphene by a factor of 20%, 23%, and 27% for C6O1, C6O2, and C6O3, respectively, indicating a monotonic degradation with respect to epoxidation. However, there is no clear trend for Poisson's ratio, implying that the local atomic configurations are dominant over oxygen concentrations in determining the Poisson ratio. Our computed high order elastic constants are good for the design of graphene oxide based flexible transparent electronics.

 Please wait while we load your content...
Please wait while we load your content...