Photochemistry of nitrate chemisorbed on various metal oxide surfaces†
Abstract
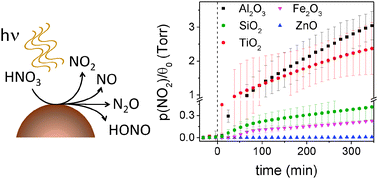
Atmospheric aerosols are known to provide an important surface for gas–solid interfaces that can lead to heterogeneous reactions impacting tropospheric chemistry. In this work, α-Fe2O3, TiO2, γ-Al2O3, SiO2 and ZnO, common components of atmospheric aerosols, served as models to investigate the gas–solid interface of nitric acid with aerosols in the presence of simulated solar radiation. Adsorbed nitrate and gaseous products can be continuously monitored with infrared spectroscopy (IR). Kinetic studies of adsorbed species were carried out using attenuated total reflectance infrared spectroscopy (ATR-FTIR). Ex situ simultaneous infrared spectroscopy of gas-phase products using a 2 m long path cell allowed the detection of gaseous products at early stages of the heterogeneous photochemical reaction. In addition, photoactive gaseous products, such as HONO, were detected as gas analysis was carried out outside the region of irradiation. All reactions were found to be first order with respect to adsorbed nitric acid and yielded gas-phase products such as NO, NO2, N2O4, N2O, and HONO. While the correlation between semiconductor properties of the metal oxide and the heterogeneous photochemical rate constant (j) is not direct, the semiconductor properties were found to play a role in the formation of relatively high proportions of greenhouse gas nitrous oxide (N2O).

 Please wait while we load your content...
Please wait while we load your content...