Combined experimental and computational NMR study of crystalline and amorphous zeolitic imidazolate frameworks†
Abstract
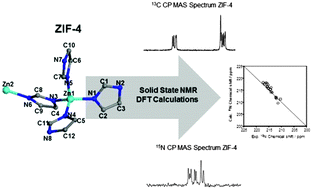
Zeolitic imidazolate frameworks (ZIFs) have attracted great interest in recent years due to their high chemical and thermal stability with promising applications in gas storage and separations. We investigate the structures of three different crystalline ZIFs – ZIF-4, ZIF-8, ZIF-zni – and their amorphous counterparts using high field 13C and 15N CP MAS NMR. The high field (20 T) allows for the observation of all crystallographically independent carbon and nitrogen atoms in the crystalline ZIFs. Combining our experimental results with density functional theory calculations enabled the assignment of all chemical shifts. The crystalline spectra reveal the potential of high field NMR to distinguish between two ZIF polymorphs, ZIF-4 and ZIF-zni, with identical [Zn(C3H3N2)2] chemical compositions. 13C and 15N CP MAS NMR data obtained for the amorphous ZIFs clearly showed signal broadening upon amorphization, confirming the retention of chemical composition and the structural similarity of amorphous ZIF-4 and ZIF-zni. In the case of amorphous ZIF-8, we present evidence for the partial de-coordination of the 2-methyl imidazole linker.
- This article is part of the themed collection: In celebration of Tony Cheetham’s 70th birthday

 Please wait while we load your content...
Please wait while we load your content...