Study on the temperature-dependent coupling among viscosity, conductivity and structural relaxation of ionic liquids
Abstract
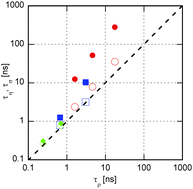
The frequency-dependent viscosity and conductivity of three imidazolium-based ionic liquids were measured at several temperatures in the MHz region, and the results are compared with the intermediate scattering functions determined by neutron spin echo spectroscopy. The relaxations of both the conductivity and the viscosity agree with that of the intermediate scattering function at the ionic correlation when the relaxation time is short. As the relaxation time increases, the relaxations of the two transport properties deviate to lower frequencies than that of the ionic structure. The deviation begins at a shorter relaxation time for viscosity than for conductivity, which explains the fractional Walden rule between the zero-frequency values of the shear viscosity and the molar conductivity.

 Please wait while we load your content...
Please wait while we load your content...