Influence of relative humidity on heterogeneous kinetics of NO2 on kaolin and hematite†
Abstract
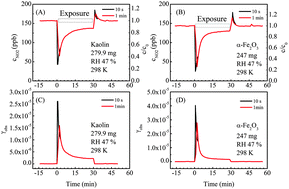
In order to obtain reliable kinetic parameters, it is required to measure the reaction kinetics of important heterogeneous reactions at ambient relative humidity (RH). In this study, the uptake coefficients and HONO yields for the heterogeneous reaction of NO2 on kaolin and hematite were measured at RH from 7% to 74% and at ambient pressure in the dark using a coated-wall flow tube reactor. The initial true uptake coefficient (γt,ini) of NO2 at RH 7% was measured to be (1.44 ± 0.10) × 10−7 and (1.58 ± 0.13) × 10−6 on kaolin and hematite, respectively, while it decreased notably on both minerals, accompanied by an increase of HONO yields, as RH increased. The average γt,ini at 32–74% RH was (4.42 ± 1.17) × 10−8 and (2.83 ± 0.84) × 10−7 on kaolin and hematite, respectively. The corresponding mean HONO yield was (36.0 ± 16.1)% and (75.9 ± 3.32)%, respectively.

 Please wait while we load your content...
Please wait while we load your content...