Redefining the established understanding of excitation dynamics of photochromic oxazines
Abstract
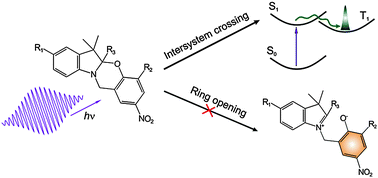
Since the introduction of photochromic indolo-benzoxazines, difference absorption of these photoexcited compounds has been assigned to the ground state of the ring-opened isomer. This assignment relies on the alleged resemblance of the spectra of photo- and chemically induced forms. In this paper, we expose the issue of the discrepancy between the absorption spectra of photoproducts and the corresponding chemically opened forms. As a result, a substantial change in the current explanation of photodynamics of photochromic oxazines is proposed. The spectral features earlier ascribed to the photoproduct are suggested to arise due to the absorption of the triplet state. This hypothesis was tested and confirmed in acetonitrile by measuring the effect of oxygen quenching of photoproduct states. In view of this interpretation, light-induced ring opening does not occur in indolo-benzoxazines dissolved in acetonitrile, and, consequently these molecules should no longer be regarded as molecular switches. On the other hand, we show that methanol solution under UV light does produce small amounts of ring-opened form of the molecule.

 Please wait while we load your content...
Please wait while we load your content...