Single Pt atom stabilized on nitrogen doped graphene: CO oxidation readily occurs via the tri-molecular Eley–Rideal mechanism†
Abstract
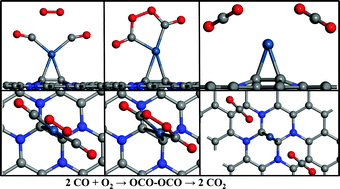
Single-atom catalysts, especially with single Pt atoms, have attracted more and more attention due to their high catalytic activity for CO oxidation. The outstanding stability and catalytic activity of a single Pt atom supported on nitrogen doped graphene (Pt/NG) are revealed using first-principles calculations. We find that the stability of a Pt atom on the NG can be promoted by picking an appropriate doping configuration. The exceptionally stable Pt/NG catalyst exhibits excellent catalytic activity for CO oxidation via a new tri-molecular Eley–Rideal mechanism (2CO + O2 → OCO–OCO → 2CO2) with an energy barrier of 0.16 eV for the rate-limiting step of OCO–OCO dissociation, which is more preferable than the other two normal Langmuir–Hinshelwood and Eley–Rideal mechanisms.

 Please wait while we load your content...
Please wait while we load your content...