Vibrational spectroscopy and theory of Fe2+(CH4)n (n = 1–3)†
Abstract
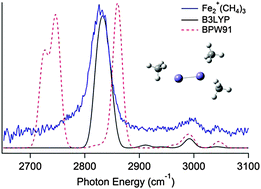
Vibrational spectra are measured for Fe2+(CH4)n (n = 1–3) in the C–H stretching region (2650–3100 cm−1) using photofragment spectroscopy, by monitoring the loss of CH4. All of the spectra exhibit an intense peak corresponding to the symmetric C–H stretch around 2800 cm−1. The presence of a single peak suggests a nearly equivalent interaction between the iron dimer and the methane ligands. The peak becomes slightly blue shifted as the number of methane ligands increases. Density functional theory calculations, B3LYP and BPW91, are used to identify possible structures and predict the spectra. Results suggest that the methane(s) bind in a terminal configuration and the complexes are in the octet spin state.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...