Triple decker sandwiches and related compounds of the first row transition metals with cyclopentadienyl and hexafluorobenzene rings: remarkable effects of fluorine substitution†
Abstract
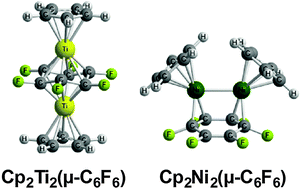
The complete series of Cp2M2(μ-C6F6) (M = Ti, V, Cr, Mn, Fe, Co, Ni) structures have been examined theoretically for comparison with their unsubstituted Cp2M2(μ-C6H6) analogues. The singlet triple decker sandwich titanium complex Cp2Ti2(η6,η6-C6F6) with a closed shell electronic structure and a non-planar C6F6 ring is preferred energetically by a wide margin (>20 kcal mol−1) over other isomers and spin states. This is in contrast to the hydrogen analogue for which related triplet spin state structures are clearly preferred. A similar low-energy triple-decker sandwich Cp2V2(η6,η6-C6F6) structure is found for vanadium but with a quintet spin state. The later transition metals from Cr to Ni energetically prefer the so-called “rice-ball” cis-Cp2M2(μ-C6F6) structures with varying hapticities of metal-ring bonding, a range of formal orders of metal–metal bonding, and varying spin states depending on the metal atom. Thus the lowest energy Cp2Cr2(μ-C6F6) structures are triplet and quintet structures with pentahapto–trihapto η5,η3-μ-C6F6 rings and formal Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cr double bonds. This contrasts with the structure of Cp2Cr2(μ-C6H6) having a bis(tetrahapto) η4,η4-C6H6 ring and a formal Cr–Cr quadruple bond. The lowest energy Cp2Mn2(μ-C6F6) structures are trans and cis quintet spin state structures. This contrasts with Cp2Mn2(μ-C6H6) for which a closed-shell singlet triple decker sandwich structure is preferred. The lowest energy Cp2Fe2(μ-C6F6) structure is a triplet cis structure with a tetrahapto–dihapto η4,η2-μ-C6F6 ring and a formal Fe–Fe single bond. The lowest energy Cp2Co2(μ-C6F6) structures are singlet spin state structures with formal M–M single bonds and either bridging bis(trihapto) η3,η3-C6F6 or tetrahapto–dihapto η4,η2-C6F6 rings. For Cp2Ni2(μ-C6F6) low energy singlet cis and trans structures are both found. The singlet cis-Cp2Ni2(μ-C6F6) structure has a Ni–Ni single bond of length ∼2.5 Å and a bridging bis(dihapto) η2,η2-C6F6 ligand with an uncomplexed C
Cr double bonds. This contrasts with the structure of Cp2Cr2(μ-C6H6) having a bis(tetrahapto) η4,η4-C6H6 ring and a formal Cr–Cr quadruple bond. The lowest energy Cp2Mn2(μ-C6F6) structures are trans and cis quintet spin state structures. This contrasts with Cp2Mn2(μ-C6H6) for which a closed-shell singlet triple decker sandwich structure is preferred. The lowest energy Cp2Fe2(μ-C6F6) structure is a triplet cis structure with a tetrahapto–dihapto η4,η2-μ-C6F6 ring and a formal Fe–Fe single bond. The lowest energy Cp2Co2(μ-C6F6) structures are singlet spin state structures with formal M–M single bonds and either bridging bis(trihapto) η3,η3-C6F6 or tetrahapto–dihapto η4,η2-C6F6 rings. For Cp2Ni2(μ-C6F6) low energy singlet cis and trans structures are both found. The singlet cis-Cp2Ni2(μ-C6F6) structure has a Ni–Ni single bond of length ∼2.5 Å and a bridging bis(dihapto) η2,η2-C6F6 ligand with an uncomplexed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. The singlet trans-Cp2Ni2(μ-C6F6) structure has a bis(trihapto) η3,η3-C6F6 ligand.
C double bond. The singlet trans-Cp2Ni2(μ-C6F6) structure has a bis(trihapto) η3,η3-C6F6 ligand.

 Please wait while we load your content...
Please wait while we load your content...