Why water makes 2-aminopurine fluorescent?†
Abstract
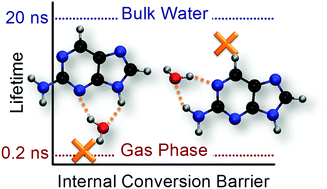
2-Aminopurine (2AP) is often chosen as a fluorescent replacement for purine bases and used as a probe in nucleic acid research. The luminescence of this molecule is strongly dependent on the environment. Through computational simulations of isolated 2AP and a series of 2AP–water clusters, we show that the experimentally-observed dependence of the excited-state lifetime of 2AP on the number and location of water molecules is controlled by a barrier for internal conversion between the S1 minimum and a conical intersection. Other possible competing pathways (proton transfer, intersystem crossing, and internal conversion at other intersections) were also investigated but discarded. The tuning of the luminescence of 2AP by water is related to the order of the nπ* and ππ* states. When a water molecule interacts with the amino group, the pathway from the S1 minimum to the conical intersection requires a nonadiabatic change, thus increasing the energy barrier for internal conversion. As a consequence, a single water molecule hydrogen-bonded to the amino group is sufficient to make 2AP fluorescent.

 Please wait while we load your content...
Please wait while we load your content...